

Which of the following is the word equation of photosynthesis? This reaction takes place in the mitochondria present in cells. In respiration, glucose and oxygen are used to produce carbon dioxide and water. (A) glucose + oxygen → carbon dioxide + water carbon dioxide + water → glucose + oxygen.glucose + water → carbon dioxide + oxygen.carbon dioxide + oxygen → glucose + water.glucose + oxygen → carbon dioxide + water.Which of the following is the word equation of respiration? Water can be decomposed, using electricity, to produce hydrogen and oxygen. Which of the following is the word equation of electrolysis of water? In order for rusting to occur, water and oxygen must be present.
When carbon is burnt in the presence of oxygen, carbon dioxide is formed. Respiration requires oxygen and glucose, to produce carbon dioxide and water. Oxidation is a process in which a substance reacts with oxygen. Which of the following examples is not oxidation? Oxygen is a product of photosynthesis, instead of a reactant. Photosynthesis is the process in which green plants make food in the presence of light. Heating of copper(II) carbonate is known as thermal decomposition, producing copper(II) oxide and carbon dioxide. When silver iodide is exposed to light, it decomposes to form silver and iodine. Which of the following examples is combustion?Ĭombustion refers to the combination of a substance with oxygen in the presence of heat, producing one or more new substances.īurning of a piece of paper requires the presence of oxygen, which in turn produces soot (carbon), carbon dioxide and water. Here are some questions for us to look into on the objectives of this article. A common example involving the mixing of substances is neutralisation, where an acid is reacted with a base to form salt and water. It also prevents the metal from rusting.Ĭhemical Change Involving The Mixing Of Two Or More SubstancesĬhemical changes can also involve the mixing of substances. It is often used in the making of coins, where a thin layer of metal is coated on the coins to make them more attractive. It can be used to break down water into hydrogen and oxygen.Įlectroplating is the process in which a substance is coated with a metal with a passage of an electric current. Photosynthesis is the process in which green plants make food in the presence of light.Įxamples of chemical changes involving electricity include electrolysis and electroplating.Įlectrolysis is the chemical decomposition of substances with the passage of an electric current. A lot of energy is also released in the process, which can be used to move vehicles.Ĭellular respiration is the process in which living cells of plants and animals take in oxygen to release the energy stored in glucose.Īn example of a chemical change involving light is photosynthesis. When petrol undergoes combustion, carbon dioxide and water vapour are released. Examples of oxidation include combustion and cellular respiration.Ĭombustion refers to the combination of a substance with oxygen in the presence of heat, producing one or more new substances. Upon the decomposition of copper(II) carbonate, in the presence of heat, copper(II) oxide and carbon dioxide are formed. Thermal decomposition is a process in which a substance is broken down into two or more simpler substances by the effect of heat. There are four factors that can bring about chemical changes:Īn example of a chemical change involving heat is the thermal decomposition of copper(II) carbonate. Here is an example of a word equation representing photosynthesis: Product(s) is/are substance(s) present at the end of the chemical reaction.Ĭondition(s) is/are written on top or below the arrow, where they are factors that are required in order for the reaction to take place. Reactant(s) is/are substance(s) present at the start of the chemical reaction.


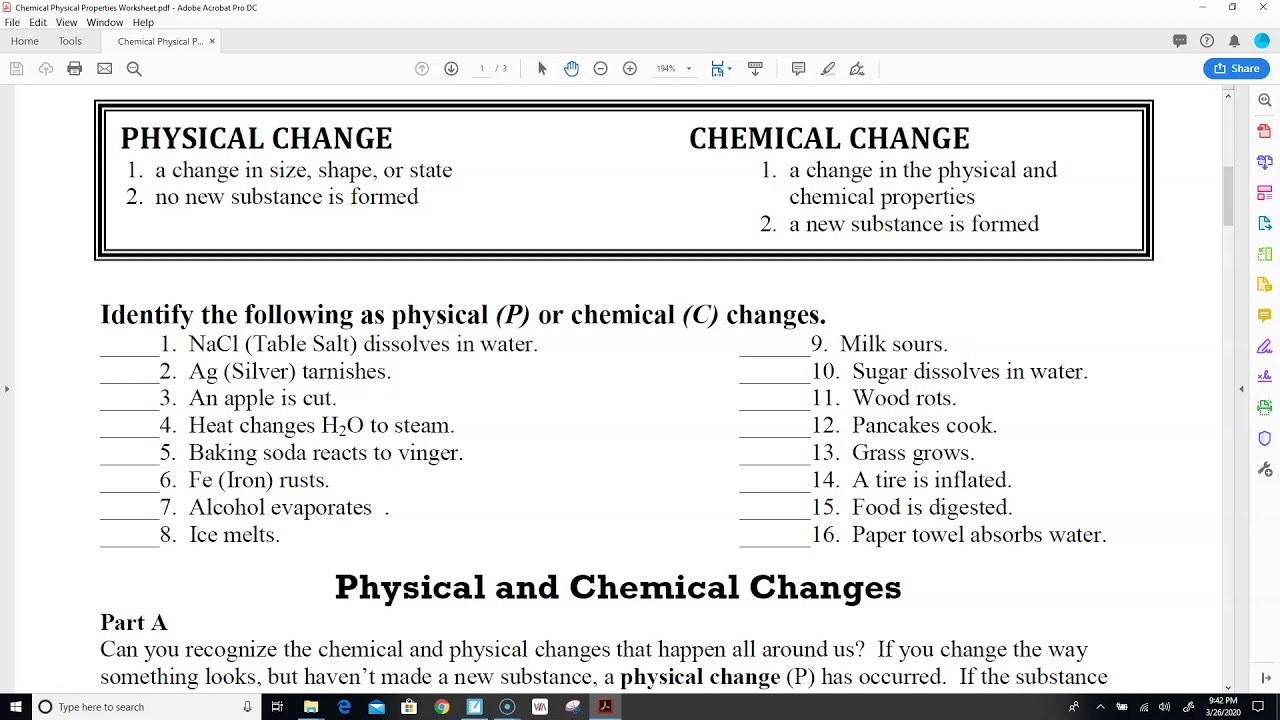
change in physical state of matter, such as melting, boiling, evaporation, condensation, freezingĬhemical changes are irreversible and involve the formation of new substances.Here are some examples of physical changes: Physical changes are reversible and do not involve the formation of new substances. Describe the different types of chemical changes.Understand how chemical changes can be represented in word equations.Understand what physical changes and chemical changes are.In the article, the following are the objectives that will be discussed.


 0 kommentar(er)
0 kommentar(er)
